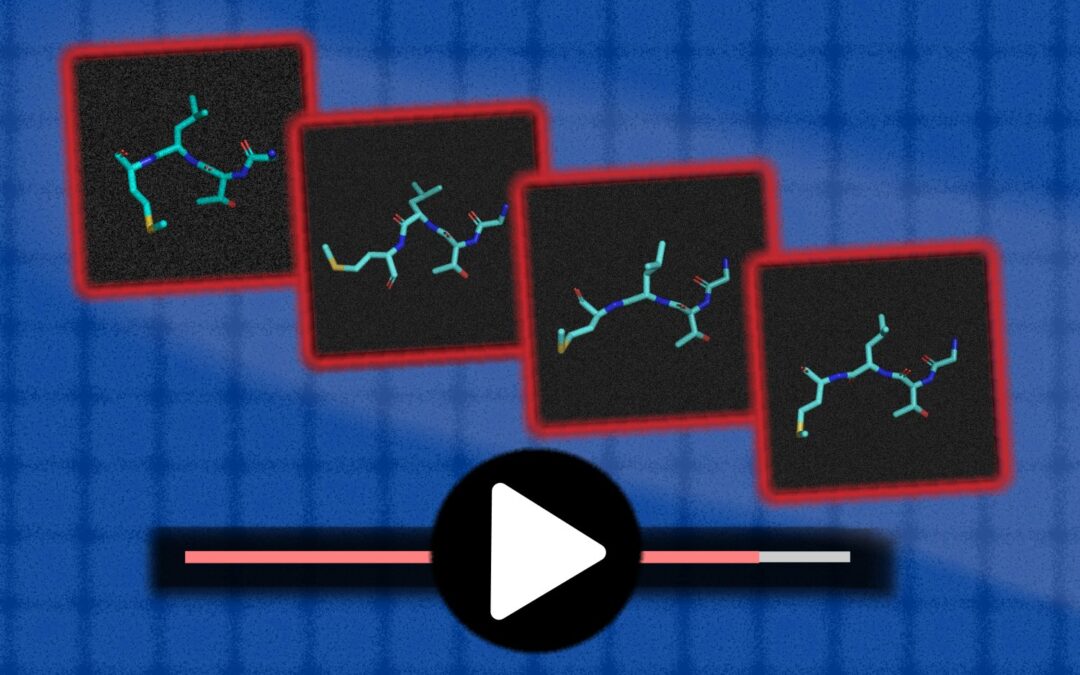
As the capabilities of generating AI models grow, you may have seen how they convert simple text cues to high-reality images or even extended video clips.
Recently, the generated AI has potential to help chemists and biologists explore static molecules such as proteins and DNA. Models like Alphafold can predict molecular structures to accelerate drug discovery, for example, MIT-assisted “Rfdiffusion” can help design new proteins. However, one challenge is that molecules are constantly moving and beating, which is important for modeling when building new proteins and drugs. Using physics, a technique called molecular dynamics, to simulate these actions on a computer can be very expensive and require time steps on billions of supercomputers.
To simulate these behaviors more effectively, MIT’s Computer Science and Artificial Intelligence Laboratory (CSAIL) and math researchers have developed a generative model to learn from previous data. The team’s system, called MDGEN, can take a framework of 3D molecules and simulate what will happen next, such as videos, connecting individual still images, and even filling in missing frames. By clicking the “play button” on the molecule, the tool can potentially help chemists design new molecules and closely study their drug prototypes on cancer and other diseases interact with the molecular structures they intend to affect.
Co-leading author Bowen Gee Shi’22 said MDGEN is an early proof of the concept, but it hints at the beginning of exciting new research directions. “Early, the generated AI models made simple videos like blinking or dogs swaying on their tails,” said Jing, a Ph.D. student at Csail. “Fast forward a few years ago, and now we have amazing models like Sora or Veo that can be useful in all sorts of interesting ways. We want to instill a similar vision into the molecular world, where dynamic trajectories are videos.”
MDGEN represents a paradigm shift with previous comparable works that generate AI, in a way that can be more broadly, the researchers say. The previous approach was “automatic regression,” meaning they rely on the previous static framework to build the next, creating a video sequence from the first frame. Instead, MDGEN generates frameworks in parallel with diffusion. This means that MDGEN can be used for, for example, to connect frames at endpoints, or “upsample” can be “upgraded” in addition to pressing playback on the initial frame.
This work was introduced in a paper presented at the Neural Information Processing Systems (Neural) Conference last December. Last summer, it was awarded for its potential commercial impact at an international conference at the ML4LMS workshop on machine learning.
Some small steps in molecular dynamics
In the experiment, Jing and his colleagues found that MDGEN’s simulations were similar to running physics simulations directly, while producing trajectories were 10 to 100 times faster.
The team first tested their model’s ability to ingest 3D frames of molecules and generated the next 100 nanoseconds. Their system pieced together 10 nanosecond blocks to achieve that duration. The team found that MDGEN was able to compete with the accuracy of the baseline model while completing the video generation process in about one minute, and only the baseline model could simulate the same dynamic for three hours.
MDGEN also models the steps between the two given the first and last frames of a nanosecond sequence. The researchers’ system exhibits a level of realism in over 100,000 different predictions: it is more likely to simulate molecular trajectories than its benchmark on the clip than on a 100-nanosecond clip. In these tests, MDGEN also indicated that it was able to generalize peptides that had never been seen before.
MDGEN’s capabilities also include simulating frames within the framework, “improving” steps between each nanosecond to capture molecular phenomena more fully faster. It can even “molecular” structures “paint” and can also restore information about them. These characteristics can ultimately be designed by researchers to design proteins based on specifications of how different parts of molecules should move.
Play with protein dynamics
MDGEN is an early sign of more efficient production of molecular dynamics advances, said Hannes Stärk, a co-leader. Nevertheless, they lack data that enables these models to immediately affect the effects of drugs or molecules that action chemists want to see in the target structure.
The researchers’ goal is to expand MDGEN from modeling molecules to predict how the protein will change over time. “Currently, we are using a toy system,” said Stärk, a PhD student at Csail. “To enhance MDGEN’s predictive power for protein modeling, we need to be based on the architecture and data currently available. We do not have a YouTube-scale repository for these types of simulations, so we want to develop a separate machine learning approach that can accelerate the data collection process for our model.”
For now, MDGEN proposes an encouraging way forward in modeling molecular changes that are invisible to the naked eye. Chemists can also use these simulations to conduct more in-depth research on medical prototypes of diseases such as cancer or tuberculosis.
“The machine learning approaches learned from physical simulations represent an emerging field of AI,” said Bonnie Berger, MIT Simons professor of mathematics, principal investigator of CSAIL and senior author on the paper. “MDGEN is a multifunctional multifunctional modeling framework that connects these two domains, and we are excited to share our early models in this direction.”
“Realistic transition path sampling between molecular states is a major challenge,” MIT Thomas Siebel Professor of Electrical Engineering and Computer Science, Institute of Data, Systems and Society, and Principal Investigator at CSAIL. “This early work demonstrates how we can address such challenges by shifting generative modeling to a complete simulation run.”
Researchers across the field of bioinformatics foreshadowed the system’s ability to simulate molecular transformation. “The molecular dynamics simulation of the MDGEN model is a joint distribution of structural embeddings, thus capturing molecular motion,” said Simon Olsson, associate professor at Chalmers Technical University. “Using masked learning objectives, MDGEN can achieve innovative use cases, such as transition path sampling, that will analogize the trajectories that are connected to stable stages.”
The National Institute of General Medical Sciences, the U.S. Department of Energy, the National Science Foundation, Machine Learning for Machine Discovery and Synthesis Consortium, the Abdul Latif Jameel Clinic’s Health Machine Clinic, the Health Threats Agency and the Defense Advanced Research Projects Agency, supported the work of MDGEN to some extent.

 1005 Alcyon Dr Bellmawr NJ 08031
1005 Alcyon Dr Bellmawr NJ 08031